Beware of reactive pans and be afraid of the lasagna cell.
Reactive pots and pans made of aluminum, cast iron, hammered steel, brass, or copper can react with some chemicals in foods, especially the acids and salts in sauces, brines, and marinades, and they can undergo a chemical reaction and create off flavors, and in rare cases, are toxic.
Non-reactive containers made of stainless steel, glass, porcelain, and enamel will not change when subjected to foods. Plastic is also non-reactive, but it can also absorb flavors and be stained by sauces.
Perhaps the most extreme example is the lasagna cell. Lasagna lovers often are appalled when they open the fridge or oven and find holes in the foil on top of the pan and black spots on their dinner.
They are not alone. Cooks who put meat in a marinade in a steel pan and cover it with aluminum foil overnight can wake up the next morning horrified to see that the foil has holes in it and cry for help in our comments section.
Sometimes barbecue cooks who use the “Texas crutch“, a technique of wrapping their meat in aluminum foil to combat a phenomenon known as “the stall” (when evaporation from the meat cools its surface and stops it from cooking), are shocked to find holes in the foil and juices leaking out when they use a steel pan and foil for the crutch.
What has happened is an electrochemical reaction called galvanic corrosion, dissimilar metal reaction.
For a better understanding of the process, I asked the AmazingRibs.com science advisor Prof. Greg Blonder. He explained that the cook has essentially created a small battery, a cell, and the electric current running through it has etched away one of the battery’s electrodes. Huh?
“All batteries consist of two electrodes, the anode and the cathode, separated by a conducting material called an electrolyte. The electrolyte carries electrons from one electrode in one direction, and waste products scavenged from the other electrode, in the other direction. A car battery has a negatively charged lead electrode (the cathode) on one side, positively charged lead oxide is the second electrode (the anode), and sulfuric acid is the electrolyte”.
So how does a pan of lasagna become a battery? “An acid such as vinegar or tomato sauce and electrically charged atoms like salt form the electrolyte. Aluminum foil is one electrode, and the pan, often steel or different alloy of aluminum, is the second electrode. This causes the aluminum foil to pit and dissolve, and you shouldn’t ingest gravy filled with metal ions”.
Blonder says “A corrosion cell is possible across two different brands of foil, between aluminum foil and a steel baking pan, and even inside a aluminum foil pouch if the acidity or temperature varies across its length. Any chemical activity difference produces an electrical current and then corrosion.”
The same thing can happen in a pan of marinade or brine. It can happen in the fridge, but the reaction occurs faster at oven temperatures. Blonder says “I always cook lasagna in a pyrex pan, and store in the fridge covered with saran first, and foil on top. Otherwise the foil will be pitted wherever it touches the tomato sauce. You may not always notice the small holes unless you hold the foil up to the light”.
And he warns the barbecue cook, “You can also form a battery between an aluminum foil wrapped piece of meat, the drippings, and a stainless steel thermometer cable. Weird but true”. Can you damage the thermometer probe by contact with foil? “Very unlikely” he says. Stainless is covered with an inert chrome oxide layer, which protects it from rusting and damage. No stain, no pain.” Click here for more on stainless steel.
What about the foil in contact with grill grates? “Generally, the grates are pretty dry when hot and will not set up a corrosion cell even if the foil leaks juices” he says. For smoking, the safest solution is to crutch in a lightly closed plastic Reynolds oven bag, butcher paper, or parchment paper, then foil. Blonder warns “Never cook in Saran Wrap. It is not designed for continuous use at boiling temperatures.”
Another option is cooking on an insulated grate such as teflon frog mats or enameled coated grates.
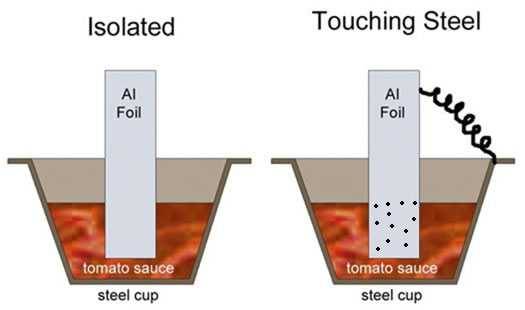
To demonstrate galvanic corrosion, Blonder filled two steel muffin tins halfway up with unadulterated pureed canned tomatoes, which is acidic. Then he suspended a 1/2″ wide strip of aluminum foil above each cup and lowered the ends of the foil into the sauce. One strip was “isolated”, that is, not directly connected to the steel. The second strip was connected to the steel cup with a short length of wire. The wire is just like the aluminum foil touching the steel pan.
After cooking in a 325°F oven for an hour, he removed the strips. They were both unaffected. He then added 1/4 teaspoon of salt to each cup, and repeated the experiment. This time, the isolated foil remained unaffected but the connected strip etched completely through at the surface, disconnecting a tab of foil (at right).

He explains that “The foil severed at the surface because the galvanic current is most concentrated at this point and the temperature is a bit higher than lower in the sauce. Once the current enters the tomato sauce, it spreads out and dissipates.
Close examination of the disconnected tab showed it was riddled with holes (shown with backlighting, below).




High quality websites are expensive to run. If you help us, we’ll pay you back bigtime with an ad-free experience and a lot of freebies!
Millions come to AmazingRibs.com every month for high quality tested recipes, tips on technique, science, mythbusting, product reviews, and inspiration. But it is expensive to run a website with more than 2,000 pages and we don’t have a big corporate partner to subsidize us.
Our most important source of sustenance is people who join our Pitmaster Club. But please don’t think of it as a donation. Members get MANY great benefits. We block all third-party ads, we give members free ebooks, magazines, interviews, webinars, more recipes, a monthly sweepstakes with prizes worth up to $2,000, discounts on products, and best of all a community of like-minded cooks free of flame wars. Click below to see all the benefits, take a free 30 day trial, and help keep this site alive.
Post comments and questions below
1) Please try the search box at the top of every page before you ask for help.
2) Try to post your question to the appropriate page.
3) Tell us everything we need to know to help such as the type of cooker and thermometer. Dial thermometers are often off by as much as 50°F so if you are not using a good digital thermometer we probably can’t help you with time and temp questions. Please read this article about thermometers.
4) If you are a member of the Pitmaster Club, your comments login is probably different.
5) Posts with links in them may not appear immediately.
Moderators